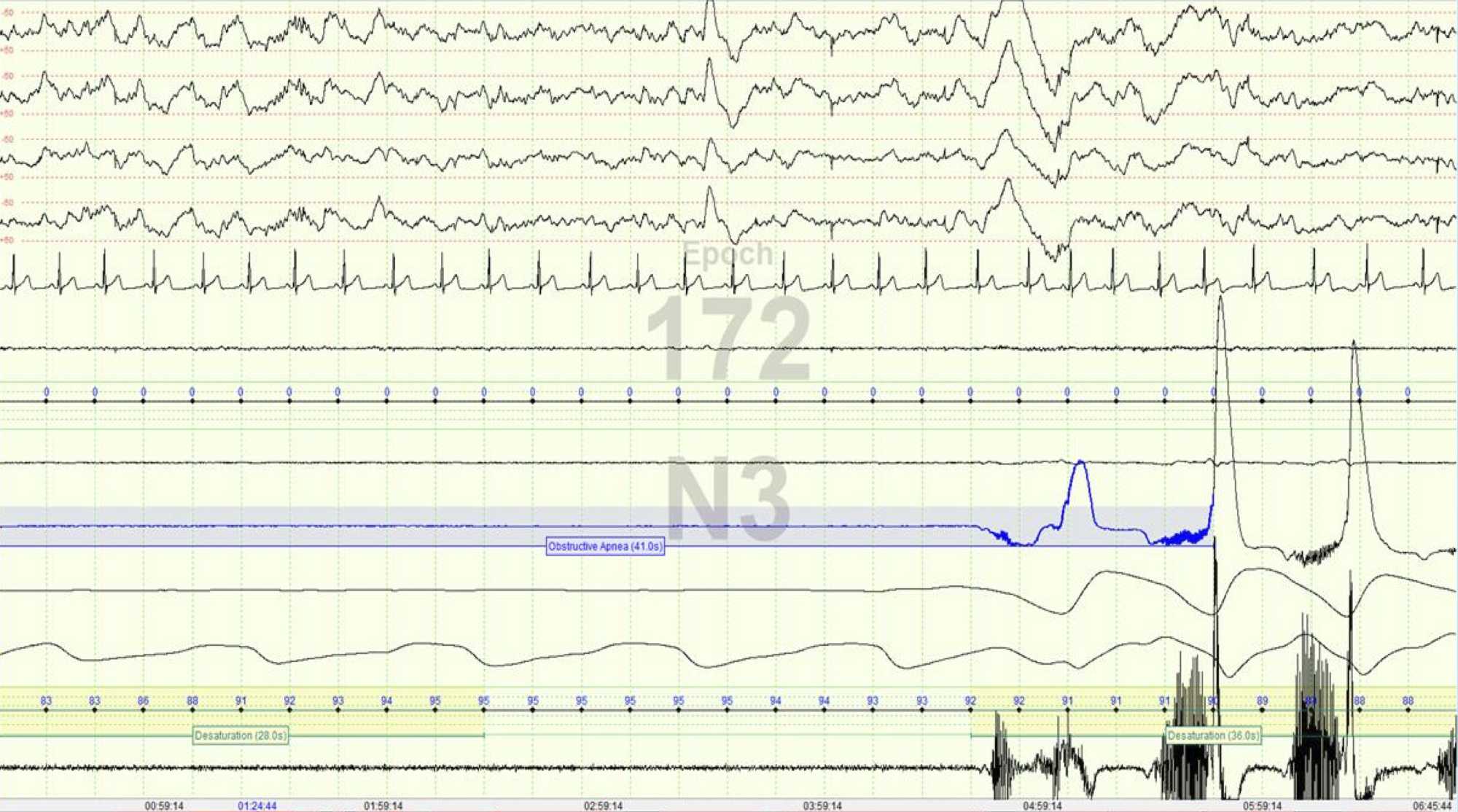
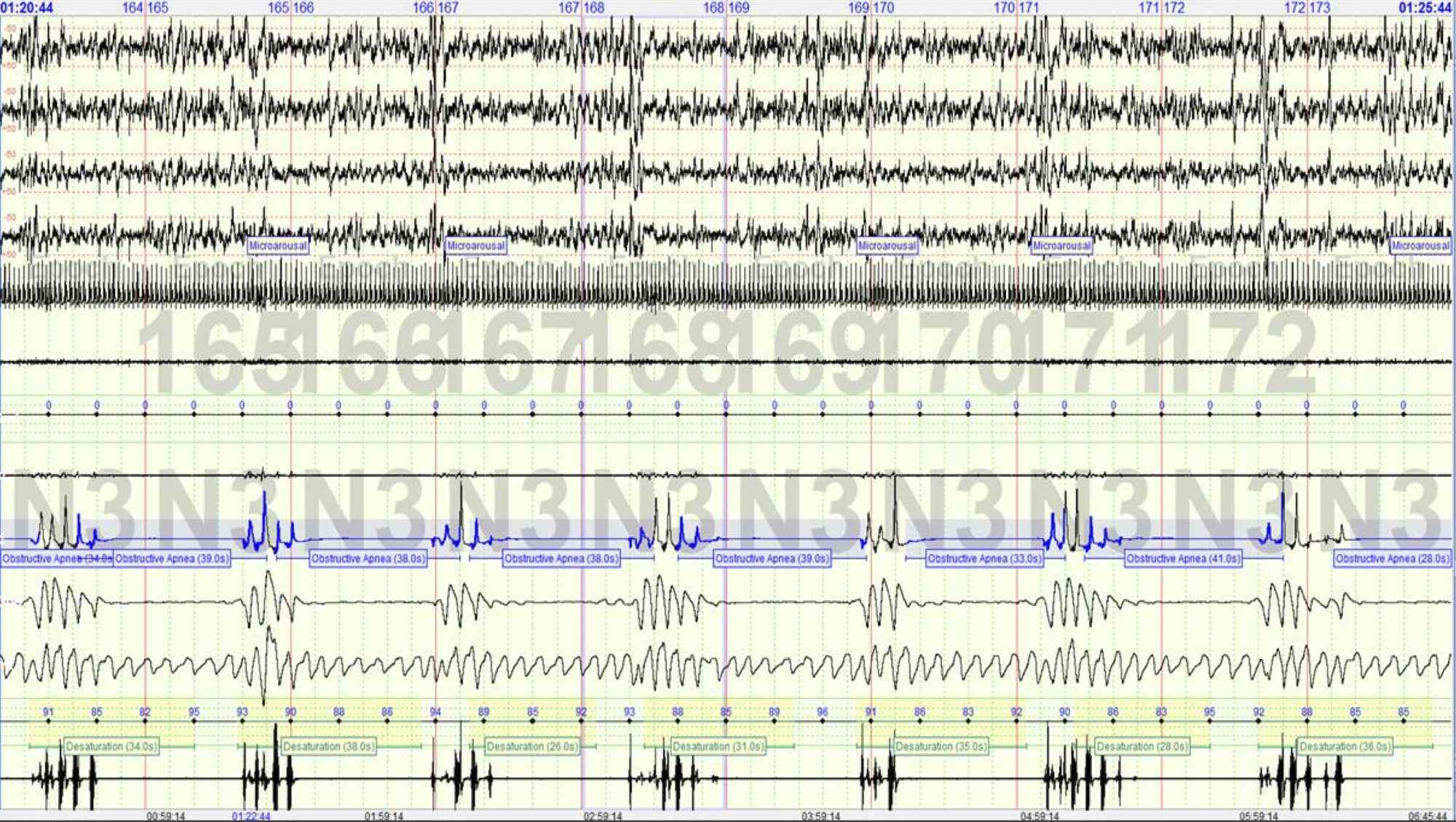
Important Clinical Parameters
Measures accurately all the sleep parameters that result from lab studies, notably it measures: sleep duration (TST), REM sleep, arousals, apnea index over the entire diagnostic range, limb movements
Additional parameters available for research only
Markers for PTSD, Markers for MDD, REM density, Spindle density, Respiratory rate, Custom analysis for research